Key Concepts
Biology
Osmosis
Cells
Chemistry
Concentration
Water transport
Introduction
Have you ever wondered how plants "drink" water from the soil? Water uptake in plants is quite complicated. A process called osmosis helps the water move from the soil into the plant roots—and then into the plant's cells. In this activity you will see for yourself how you can make water move with osmosis!
Background
Most water in the ground is not pure water. It usually contains dissolved mineral salts. Animals and plants need these salts (which include calcium, magnesium, potassium and the sodium you might be familiar with as table salt) to grow, develop and stay healthy. Different water sources carry different amounts of these salts. Nature wants to balance a system that is not balanced. So if you mix water with two different salt concentrations, the salts don't stay separated but spread out evenly through the solution until the salt concentration is the same throughout.
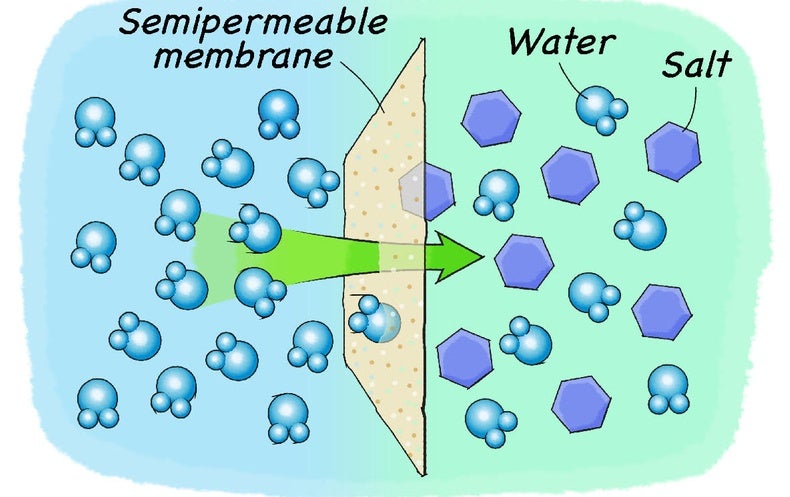
You'll find a similar reaction if you separate two salt solutions with a semipermeable membrane. A semipermeable membrane is a type of barrier that only lets certain particles pass through while blocking others. This type of membrane usually lets water pass through but not the salts that are dissolved in the water. In this situation, because only water can move through this membrane, the water will start moving from the area of lower salt concentration (which has more water and less salt) to the area of higher salt concentration (which has less water and more salt). This water movement will only stop once the salt and water concentration on both sides of the membrane is the same.
The process of moving water across a semipermeable membrane is called osmosis. Plants use this process to their advantage for water uptake. They create an environment of high salt concentration in their root cells that are in contact with the soil. The cell walls act as a semipermeable membrane that only let water through. Because the water outside the root cells has a lower salt concentration, water starts moving into the root cells due to osmosis. The water entering the plant fills up the cells and can travel to the rest of the plant. Osmosis, however, works in both directions. If you put a plant into water with a salt concentration that is higher than the concentration inside its cells, water will move out of the plant to balance out the concentration difference. As a result the plant shrinks and eventually dies. You will see this effect with your own eyes in this activity using potatoes and different saltwater solutions.
Materials
- Distilled water
- Measuring cup with milliliters (mL)
- Table salt
- Weight scale with gram measurements
- Three plastic cups or glasses
- Spoon
- At least three potatoes
- Apple corer. (Alternatively, you can have an adult help you use a cutting board and knife.)
- Knife (and an adult helper to help you use it)
- Ruler
- Paper
- Pen or pencil
- Timer
- Paper towels
- Graphing paper (optional)
- Other vegetable(s) or fruit (optional)
Preparation
- Prepare three different saltwater solutions. Create labels for the three cups: "0 grams," "2 grams" and "4 grams."
- To each of the cups add 100 mL of distilled water.
- Weigh out 2 grams of table salt, and add it to the cup that says "2 grams." Then weigh out 4 grams of table salt, and add it to the cup labeled "4 grams." Use a spoon to mix the solutions until all the salt is dissolved.
- Draw a table in which you can enter the starting measurements (length and diameter or width) and end measurements of each potato strip for every salt concentration (0, 2 and 4 grams).
- Prepare at least three potato cores. Carefully push the corer all the way through the potato, and remove the core carefully so the potato piece stays intact. (Alternatively, you can have an adult help cut the potato into strips that all have the same dimensions.) The potato pieces should be at least one-half inch thick and two inches long. (Ideally you will be able to prepare nine matching cores or strips so you can test three pieces in each solution to compare the results thoroughly.)
- Use a knife to carefully remove any potato skin from your cores, and rinse the cores quickly with water.
- Use a ruler to ensure each potato piece is the same size (ideally to the millimeter). Carefully use a knife to trim any pieces as needed.
- Measure the dimensions (length and diameter or width) of each potato strip in millimeters, and write the information in the table.
- Optionally, you can also weigh each potato piece and record their weights.
Procedure
- Put one potato strip (or three if you made nine pieces) into each of the cups. While you do that feel the potato strips with your fingers and try to flex them a little bit. How do they feel? Are they easy to bend?
- Start your timer for 30 minutes. Let the potato strips sit in the different solutions for the whole time. What do you think will happen to the strips in each of the cups?
- After 30 minutes inspect the potato strips inside the solutions. Do you see any changes?
- Take the potato strip(s) out of the "0 grams" cup and place on a paper towel. While doing that feel the potato pieces again and try to bend them slightly. How do they feel? Are they easier or more difficult to bend than before?
- Use the ruler to measure the exact length and diameter or width (in millimeters) of each of the potato strips, and write the results in your table. What do you notice about the potato strip measurements? Optionally you can weigh these pieces and record their weights.
- Next take the potato strips from the "2 grams" cup, and place them on a paper towel; as you do this feel them. Measure their lengths and diameters or widths. Write your results in the table. Optionally you can weigh these pieces and record their weights. What changed about these potato strips?
- Repeat the same steps with the potato strips in the "4 grams" cup. Write your results in the table. Are your results for these similar or different compared with the other ones?
- How did the feeling of the strips compare based on what solution they were in? Why do you think this is?
- Compare the results in your table. How did the length and diameter or width of the potato strips change in each cup? What about the weights if you took them? Can you explain your results?
- Extra: If you weighed each of your strips before and after soaking them, compare the weights. How does the mass of the potato strips change in each solution?
- Extra: Leave the potato strips in the solutions for a longer time period. How do they look if you let them soak in the saltwater for one hour or overnight?
- Extra: If you have graphing paper, make a graph of your results with the salt concentration on the horizontal axis and the potato strip length or diameter after soaking on the vertical axis. Draw two lines to make your graph. For the first, connect each of the data points you found. For the second, draw a horizontal line starting at the point on the vertical axis that shows the original length of your potato strip. Based on your graph can you find a salt concentration at which the potato strip length should not change at all?
- Extra: How does the activity work with other vegetables or fruit? Try it to find out!
Observations and Results
Did your potato strips shrink and expand? At the beginning all the potato strips should have had the same length and should have all felt the same. When you put them into the different solutions, however, this starts to change. Whereas the potato strips in the "0 gram" cup probably got larger in size, the other potato strips probably got shorter after leaving them in the saltwater for 30 minutes. (If you didn't see any significant changes after 30 minutes, leave the potato strips in the saltwater solutions longer.)
The shrinking and expanding of the potato strips is due to osmosis. Potatoes are made of cells, and their cell walls act as semipermeable membranes. The 0 grams solution contains less salts and more water than the potato cells (which have more salts and less water). To balance out these concentration differences, the water from the cup moves into the potato cells. The incoming water in the potato cells pushes on the cell walls and makes the cells bigger. As a result the whole potato strip gets bigger. The opposite is the case in the higher concentration salt solutions. If the salt concentration in the cup is higher than inside the potato cells, water moves out of the potato into the cup. This leads to shrinkage of the potato cells, which explains why the potato strips get smaller in length and diameter. Due to the shrinking of the potato cells the potato strip also becomes less rigid. If you bent the potato strips, you should have noticed that those that had been in the solution with the highest amount of salt were much easier to bend than the potato strips in the water without salt.
If you made the graph you probably noticed that there is a salt concentration at which the potato strip neither expands nor shrinks. This should be where your data curve and your start length line intersect. At this point the salt concentration inside the potato cells and inside the cup are the same. Because the concentrations are already balanced no water moves.
Cleanup
Discard the saltwater solutions in the sink. Throw the potato strips into the compost, and clean up your workspace. You can cook with the other pieces of unused potato.
More to Explore
Osmosis, from Biology Dictionary
Do Fish Drink? from McGill University's Office for Science and Society
Cucumber Chemistry: Moisture Capture with Desiccants, from Scientific American
Suck It Up! How Water Moves Through Plants, from Science Buddies
STEM Activities for Kids, from Science Buddies
"potato" - Google News
January 09, 2020 at 12:47PM
https://ift.tt/2N8VrmC
Make a Potato Shrink--with Saltwater - Scientific American
"potato" - Google News
https://ift.tt/2rh4zOj
Shoes Man Tutorial
Pos News Update
Meme Update
Korean Entertainment News
Japan News Update
Bagikan Berita Ini















0 Response to "Make a Potato Shrink--with Saltwater - Scientific American"
Posting Komentar